- Home
- Blog Entries
- Materials Up Close & Personal: SALT
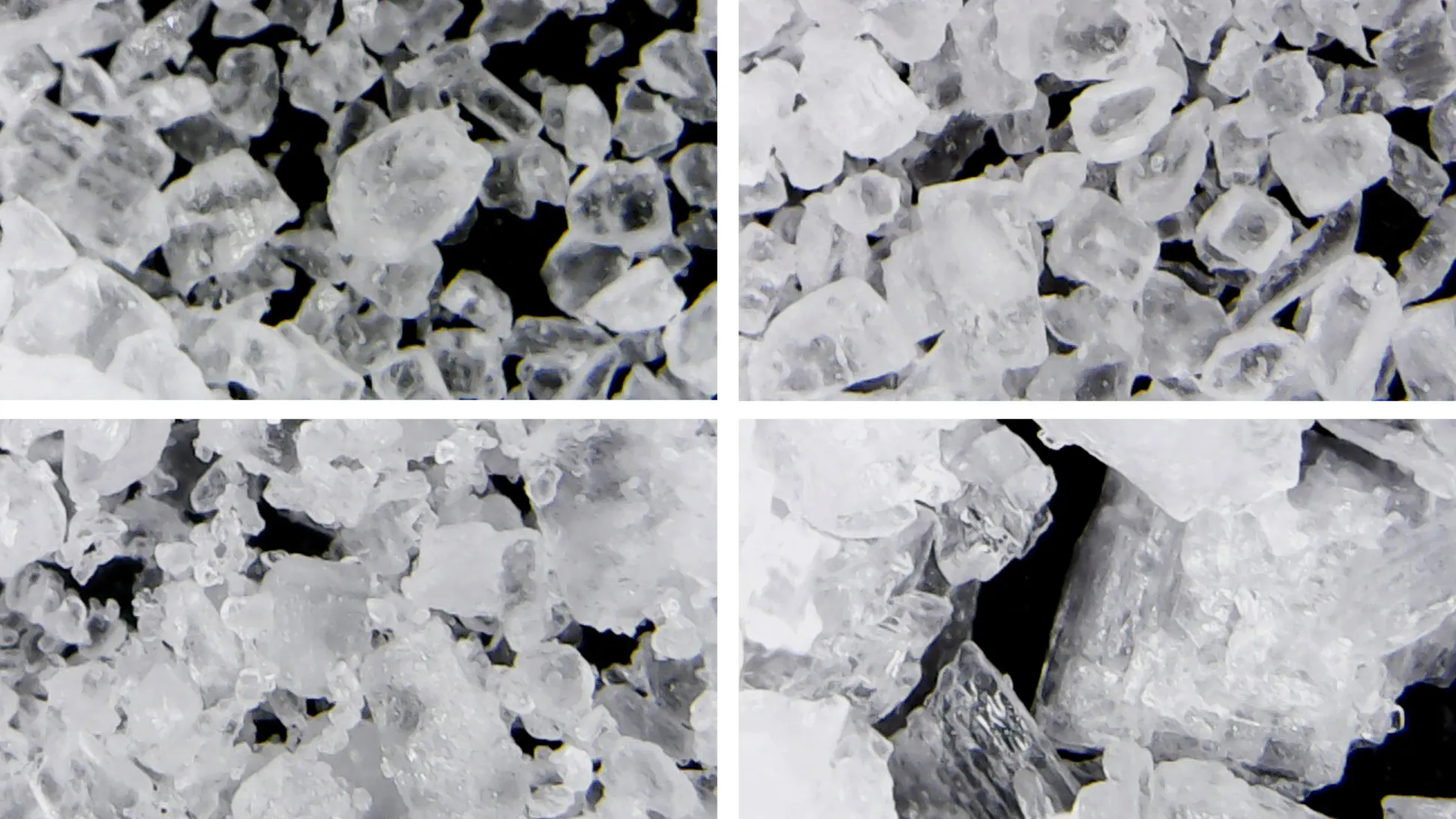
This week we look in fine-grained detail at common SALT. Simple sodium chloride, the inorganic mineral that hails from the sea and the rocks that erode into it, is not only a favourite flavouring for our food. It also plays a crucial role in food preservation, textile dyeing, ceramic glazing and soap manufacture. Salt is present in our blood, sweat and tears, and is at once essential for human life, and a danger to it.
Hundreds of millions of tons of salt are produced every year, all across the world, but with China and the US as the biggest, saltiest players. Approximately 293 million tons of the white stuff were made in 2019 by one of three processes. The first and by far the oldest method is solar evaporation from sea water in open-air salt pans, which dates back to at least the Bronze Age in the UK. The second process involves mining from underground seams of rock salt (halite) that were left behind by the evaporation of prehistoric oceans. The third method involves pumping water into those deposits to dissolve out salt and then evaporate the brine.

Only 6% of that enormous global glut of salt is used in our food, and more than half of the salt produced is used by cold countries to prevent roads from icing over in winter. The mixture of rock salt and grit used on our roads combines with water on the surface of the tarmac to produce a salty solution that has a lower melting point than pure water, meaning that it no longer freezes at zero degrees. This salty sprinkling keeps traffic moving in a blizzard, and is thought to reduce ice-induced accidents by nearly 90%, but comes with its own hazards. Road salt run-off has known environmental impacts: accumulating in waterways and killing off rainbow trout, roadside and aquatic plants, and salamanders and frogs. It is also thought to attract birds, deer, elk and moose to roads to satisfy their salt cravings, ironically increasing the risk of car vs. animal collisions.

Aside from its use in cooking, salt is central to innumerable making processes. It is an essential ingredient in the production of glossy, salt-glazed ceramics, like these distinctive, orange-peel-surfaced Bernard Leach pieces. Salt also plays a crucial role as a catalyst in textile dyeing, whereby salt’s easily dissolvable, positively-charged sodium ions (Na+) attach to the fabric, and attract the negatively-charged ions of the dye, helping to absorb and fix colour to cloth. Salt’s offspring, chlorine and caustic soda – produced by the electrolysis of brine – are essential ingredients in the manufacture of numerous other materials. Caustic soda (a.k.a. sodium hydroxide or lye) is a core ingredient in soap-making, paper-making and in the extraction of aluminium from its ore bauxite, whereas chlorine is used to treat drinking water, to make titanium dioxide pigment for white paint, and as a feedstock for PVC plastics that are prevalent in our raincoats and drainpipes.
We can also thank salt for the sausages, spams and salamis of this world. Both dry salt cures and saturated aqueous solutions of salt (brine) are used to preserve food, and are responsible for the joys of saltfish, smoked salmon, beef jerky and pickles. Salt sucks moisture out of both the food and the bacteria that live on it by the process of osmosis. The salt around the outside of the food draws water molecules out and replaces them with salt (sodium and chloride) ions until the amount of salt is equal inside and out. The same thing happens to bacteria, which become dehydrated. Because most micro-organisms cannot survive and replicate in this kind of salty environment, this slows down decomposition. Salting is one of the oldest food preservation techniques and was heavily used by the Egyptians, Romans and Gauls, continuing right up until the middle of the 20th Century, but becoming less widespread with the advent of the fridge. The Egyptians also famously used similar techniques to mummify the bodies of their pharaohs using natron, a naturally-occurring combination of sodium chloride (table salt), sodium bicarbonate (baking soda) and sodium carbonate (washing soda).
Salt is a favourite flavouring for our food, and we are not the only animals who feel that way: horses, elephants and porcupines are all partial to salt licks, and Japanese macaques have been known to dip potatoes in salt water before eating them. Some of this hankering for salt may be down to a physiological need for sodium, as it is crucial for many bodily processes, but the fact that we are not the only mammals to eat far more salt than we need suggests that this selection of salty foods also comes down to taste preference. Chemists think salt is so significant for our taste experience because it selectively filters flavour; supressing unpalatable tastes, like bitterness, and enhancing more pleasant tastes, like sweetness. Our sensitivity to, and preference for salt can vary a lot from person to person (and probably from macaque to macaque), and is affected by inherited differences and the number and effectiveness of taste receptors on our tongues, as well as by general health, age and experience.
Salt has varying levels of saltiness: the amount of sodium chloride in it can range from 98% to 99.7%. The rest is made up of other minerals and sediments that add to the salt’s flavour and appearance, like the potassium, magnesium and calcium that make Himalayan salt pink, or the ground lava, clay or coral in red and black Hawaiian salts, as well as anti-caking additives like magnesium carbonate that prevent crystals from getting damp and clumping together. Fleur de sel (literally translated as ‘flowers of salt’) is one of the saltiest salts, and is thought by chefs and chocolatiers to have one of the most complex flavours, thanks to its variety of trace minerals. This patissier’s favourite is most commonly associated with the North Atlantic coast of France, where its crystals are grown in enormous open air salt flats.
The way that salt crystals form determines their shape: whether they become solid, crunchy cuboids, or fragile, crumbly flakes. When salt is formed by the slow evaporation of seawater in an open salt pan, salt crystals form at the surface of the brine and solidify into fragile, hollow pyramids that need to be gently raked off the surface of the pool to prevent them sinking and solidifying. When salt is produced by quick evaporation in a closed tank, often under a vacuum, evaporation occurs simultaneously throughout the brine, resulting in the small regular cube-shaped crystals that become the grains of salt in a shaker.

There’s been a lot of attention paid in recent years to the perils of too much salt in our diets, with excessive consumption linked to hypertension and heart disease. However, salt is used elsewhere in the body to positive effect in cleaning wounds, irrigating snotty nostrils, removing contact lenses, and replacing lost bodily fluids when given intravenously. We need a certain amount of salt in our diets, as its component sodium and chloride ions keep our body chemistry in working balance, affecting our nervous system, muscles and blood flow. Most of the body’s sodium ions are located in plasma: the fluid portion of our blood and the liquid around our cells. If we consistently consume too much salt we produce excessive amounts of plasma, which puts too much pressure on our blood vessels, increasing our risk of heart disease and stroke. On the other hand, too little salt can also result in cardiac and other health problems.
As an essential nutrient that allows for the preservation and long-distance transportation of food, salt was massively important to ancient economies, as it is today. Despite the difficulties of tracing salt in the archaeological record (because of its solubility in water), there has been an explosion of interest in its history and prehistory since the 1970s. Even though salt itself almost never survives the ravages of time, production sites and clay vessels used for evaporation provide evidence of its manufacture and exchange between communities with and without local availability of salt. Salt’s bacteriocidal properties also help to preserve organic remains like leather and textiles, leaving us with a record of equipment used. With this evidence, researchers have shown that access to salt has played an important role in bringing communities together through trade, building the wealth and power of cities, kingdoms and empires, but also in dividing people through numerous ‘salt wars’.
The stories of many of the materials we now take for granted, like salt (and sugar, coffee, gold, diamonds…), are often inextricably linked with difficult and painful colonial legacies, where the production, exchange and consumption of everyday substances were used as instruments for establishing and maintaining power. We talk a lot about the overconsumption of salt in societies where we now consume too much processed food, but in 19th and 20th Century British India, where this essential mineral was heavily controlled and highly taxed by colonial powers, salt starvation was much more of a problem. Mahatma Gandhi’s Salt March was a pivotal moment in the campaign for Indian independence, where he walked 240 miles to the Arabian sea to disobey the British Raj’s law that Indian citizens could not collect their own salt. In picking up a pinch of natural salt from the flats, he symbolically defied British rule.
About our blog series 'Materials: Up Close & Personal'
We are publishing a new series of ten extra-long Materials Library profiles, through which our Materials Librarian Sarah Wilkes will pay homage to those silent and humble materials that we surround ourselves with daily, but that often we don’t even notice. Every week or so, she will be exploring the expansive inner lives and backstories of the surfaces, substances and stuff around us, and sharing it with our community through our blog and social media #MaterialsLibraryUpCloseAndPersonal.
We would love you to join in too! Send us a picture of one of your favourite household substances with a few words about what you’ve noticed after spending some time getting to know your material cohabitants. We will periodically post a collection of these little gems on our website.



